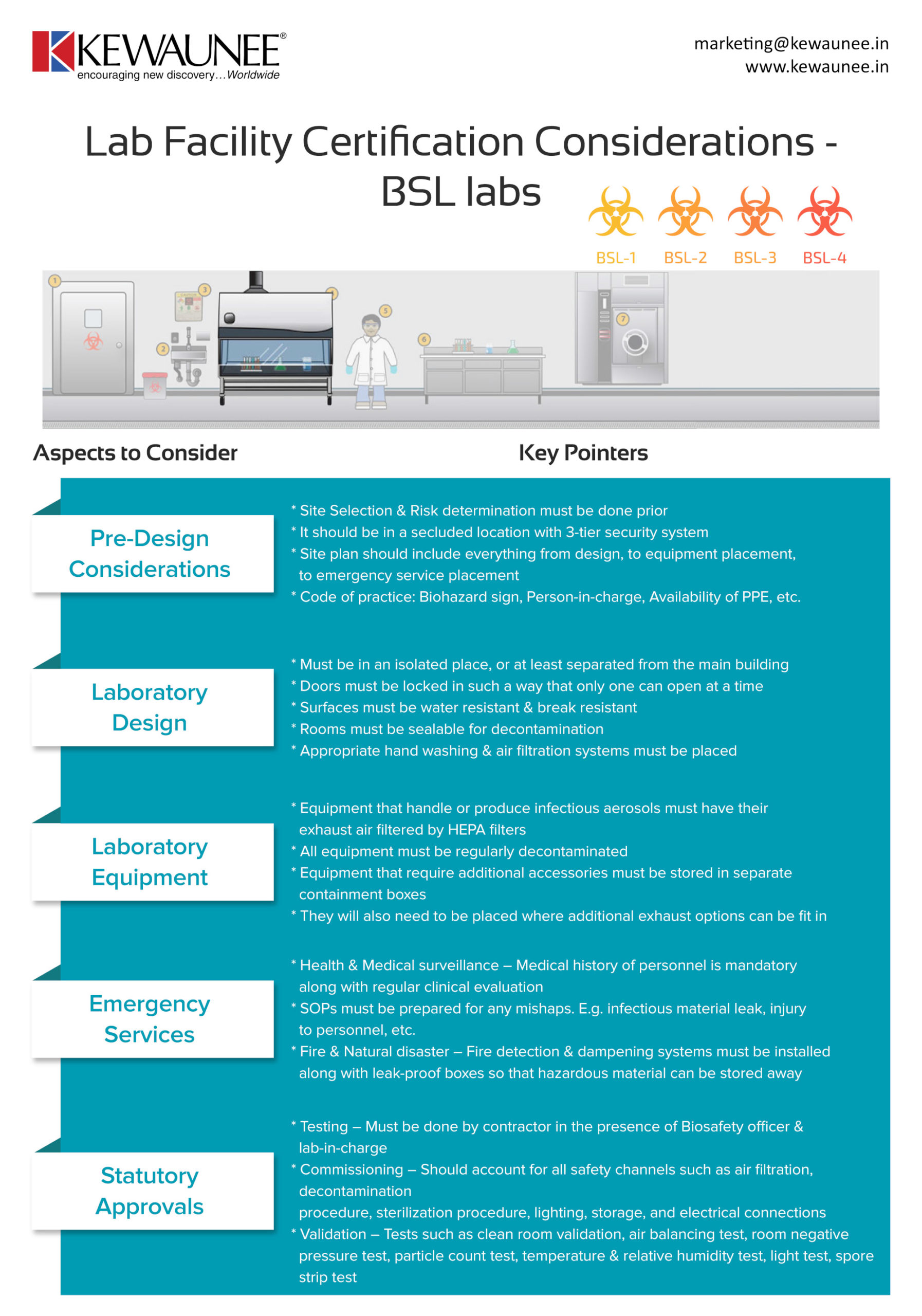
Lab Facility Certification Considerations – BSL labs
Biosafety laboratories are a key component of any infectious disease laboratory, and the pandemic has only reiterated it. It’s very important to ensure that all the compliance standards are being met prior to the designing of the laboratory. It reduces the possibility of approval or rejection and provides a streamlined structure to the process.
In this infographic, we’ve laid down certain things you need to account for in the BSL laboratory certification standards. A list of our services can be found here.
| Aspects to Consider | Key pointers |
| Pre-Design considerations |
|
| Laboratory Design |
|
| Laboratory Equipment |
|
| Emergency Services |
|
| Statutory Approvals |
|
Comments are closed.