API Manufacturing for Pharmaceutical Production
The pharmaceutical industry is continually evolving, driven by technological advancements and the need for more efficient and cost-effective solutions.
In this dynamic landscape, optimizing API (Active Pharmaceutical Ingredient) manufacturing is a top priority. APIs are the core of pharmaceutical production, as they involve the synthesis of the active components that make a drug effective.
This blog explores the cutting-edge strategies that pharmaceutical companies are using to optimize API manufacturing, from innovative processes to advanced technologies.
1. Understanding the Importance of API Manufacturing
APIs are the heart of any pharmaceutical formulation. They are the key components responsible for the therapeutic effects of a drug. Therefore, the efficiency and precision of API manufacturing directly impact the quality and safety of pharmaceutical products.
By focusing on API manufacturing optimization, pharmaceutical companies can ensure the consistent production of high-quality drugs while reducing costs.
In the past, API manufacturing was often viewed as a straightforward process. However, with the increasing complexity of drugs and the need for tailored therapies, the importance of optimizing API manufacturing cannot be overstated.
The intricacy of modern pharmaceuticals requires a deep understanding of chemical processes, and this is where cutting-edge strategies come into play.
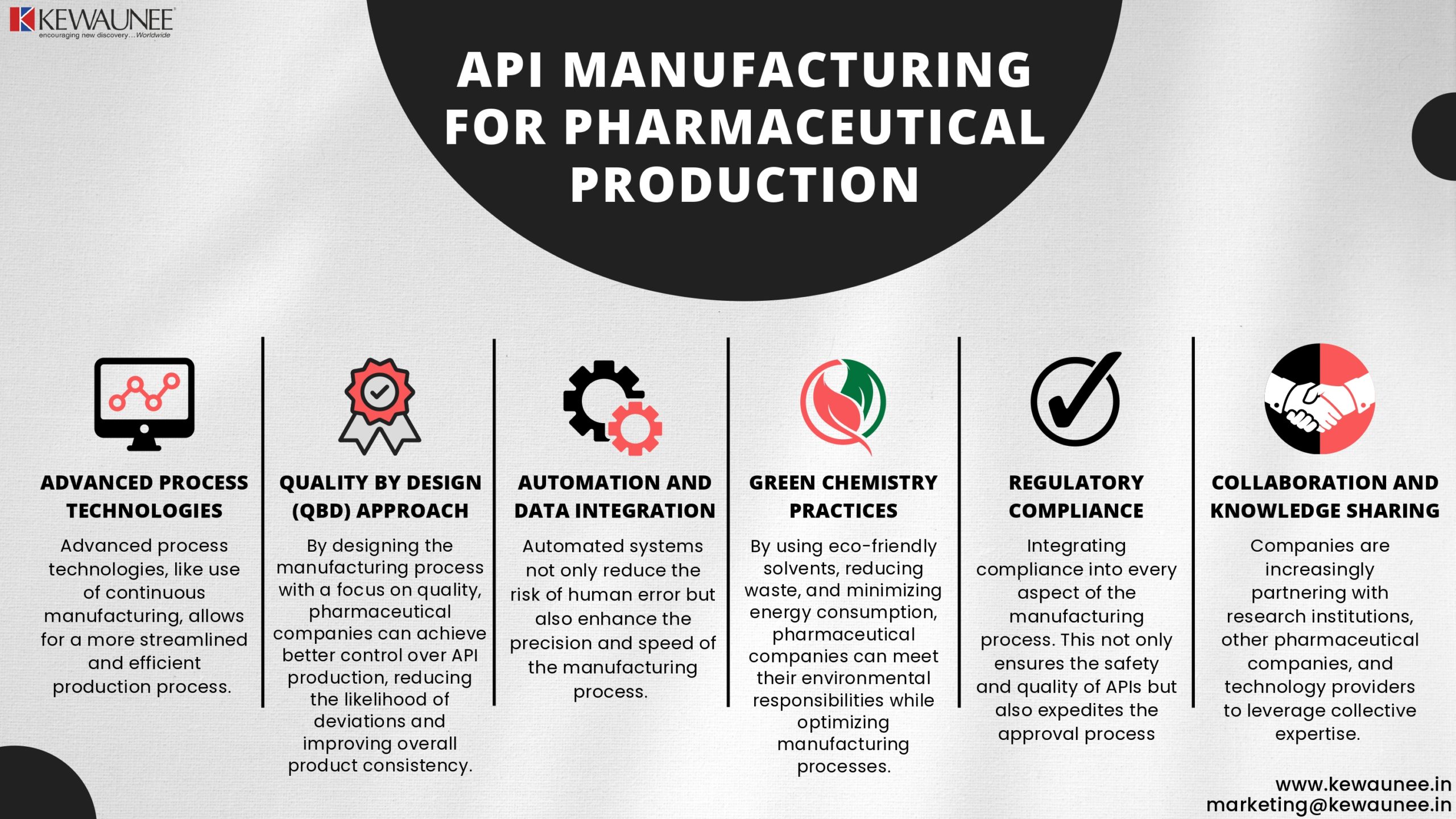
2. Advanced Process Technologies
One of the primary strategies for optimizing API manufacturing is the adoption of advanced process technologies. This includes the use of continuous manufacturing, which allows for a more streamlined and efficient production process.
Continuous manufacturing minimizes batch-to-batch variations and reduces the need for extensive quality control, resulting in higher-quality APIs and cost savings.
Continuous manufacturing represents a significant shift from traditional batch processing. It involves the uninterrupted production of APIs, which not only saves time but also reduces the risk of contamination and errors.
The real-time monitoring of processes in continuous manufacturing ensures that any deviations can be immediately addressed, maintaining the highest quality standards. This approach has gained popularity in recent years, and pharmaceutical companies are increasingly investing in the technology required to implement continuous manufacturing.
3. Quality by Design (QbD) Approach
The Quality by Design (QbD) approach is another cutting-edge strategy in API manufacturing. QbD emphasizes the systematic development of processes, ensuring that quality is built into the product from the beginning.
By designing the manufacturing process with a focus on quality, pharmaceutical companies can achieve better control over API production, reducing the likelihood of deviations and improving overall product consistency.
QbD is a comprehensive approach that involves the identification of critical process parameters and the development of a robust process that minimizes variability. By systematically understanding the factors that can impact the quality of the final product, pharmaceutical companies can optimize API manufacturing to consistently produce high-quality APIs.
4. Automation and Data Integration
Automation and data integration play a vital role in optimizing API manufacturing. Automated systems not only reduce the risk of human error but also enhance the precision and speed of the manufacturing process.
Moreover, these systems generate valuable data that can be used for real-time monitoring and process improvement, leading to more efficient API production.
In the world of API manufacturing, precision and consistency are paramount. Automated systems provide the level of control and accuracy necessary to meet these requirements. They can manage various aspects of the manufacturing process, from mixing and reaction monitoring to product analysis and packaging. This not only reduces the chances of errors but also allows for a more efficient use of resources.
The data generated by automated systems is equally crucial. It provides valuable insights into the manufacturing process, allowing for real-time adjustments and fine-tuning. The integration of data from various stages of production enables pharmaceutical companies to identify trends, detect deviations, and continuously improve the manufacturing process.
5. Green Chemistry Practices
Sustainability is a growing concern in the pharmaceutical industry, and it’s no different in API manufacturing. The adoption of green chemistry practices is a cutting-edge strategy that aims to reduce the environmental impact of API production.
By using eco-friendly solvents, reducing waste, and minimizing energy consumption, pharmaceutical companies can meet their environmental responsibilities while optimizing manufacturing processes.
Green chemistry practices are not only environmentally responsible but also economically viable. They emphasize the use of safer solvents and reagents, the reduction of hazardous by-products, and the overall minimization of waste. In API manufacturing, this approach involves the design of environmentally friendly processes that are both efficient and effective.
The pharmaceutical industry is increasingly aware of its environmental footprint, and green chemistry practices align with the global push for sustainability. By adopting these practices, pharmaceutical companies not only contribute to a healthier planet but also demonstrate their commitment to responsible manufacturing.
6. Regulatory Compliance
Adhering to stringent regulatory standards is essential in API manufacturing. Cutting-edge strategies involve integrating compliance into every aspect of the manufacturing process. This not only ensures the safety and quality of APIs but also expedites the approval process, allowing for quicker market entry of pharmaceutical products.
In the world of pharmaceuticals, regulatory compliance is non-negotiable. The approval and market entry of new drugs depend on meeting strict regulatory requirements. Therefore, pharmaceutical companies must design their API manufacturing processes with regulatory compliance in mind from the outset.
Modern pharmaceutical manufacturing facilities are designed to comply with Good Manufacturing Practices (GMP) and other relevant regulations. These facilities incorporate the latest technologies and quality control measures to ensure that every API produced meets the required standards. The integration of compliance into the manufacturing process not only expedites approvals but also reduces the likelihood of costly delays.
7. Collaboration and Knowledge Sharing
In the fast-paced world of pharmaceuticals, collaboration and knowledge sharing are indispensable. Companies are increasingly partnering with research institutions, other
pharmaceutical companies, and technology providers to leverage collective expertise. Sharing insights and innovations can lead to more effective strategies for API manufacturing optimization.
Collaboration is the driving force behind innovation in API manufacturing. The complexity of modern drugs often requires a multidisciplinary approach. By partnering with experts in various fields, pharmaceutical companies can access a broader pool of knowledge and experience.
One example of successful collaboration is the partnership between pharmaceutical companies and academic institutions. These collaborations often result in groundbreaking research and the development of novel manufacturing techniques. Additionally, partnerships with technology providers can lead to the adoption of cutting-edge equipment and processes that enhance API manufacturing efficiency.
Summary
Optimizing API manufacturing is not a static goal but a dynamic journey. By adopting these cutting-edge strategies, pharmaceutical companies can navigate the evolving landscape of pharmaceuticals and continue to deliver innovative and life-saving drugs to the world.
As technology advances and regulatory standards evolve, the pharmaceutical industry will undoubtedly face new challenges, but it is well-equipped to meet them head-on with the knowledge and strategies discussed in this blog.
Comments are closed.