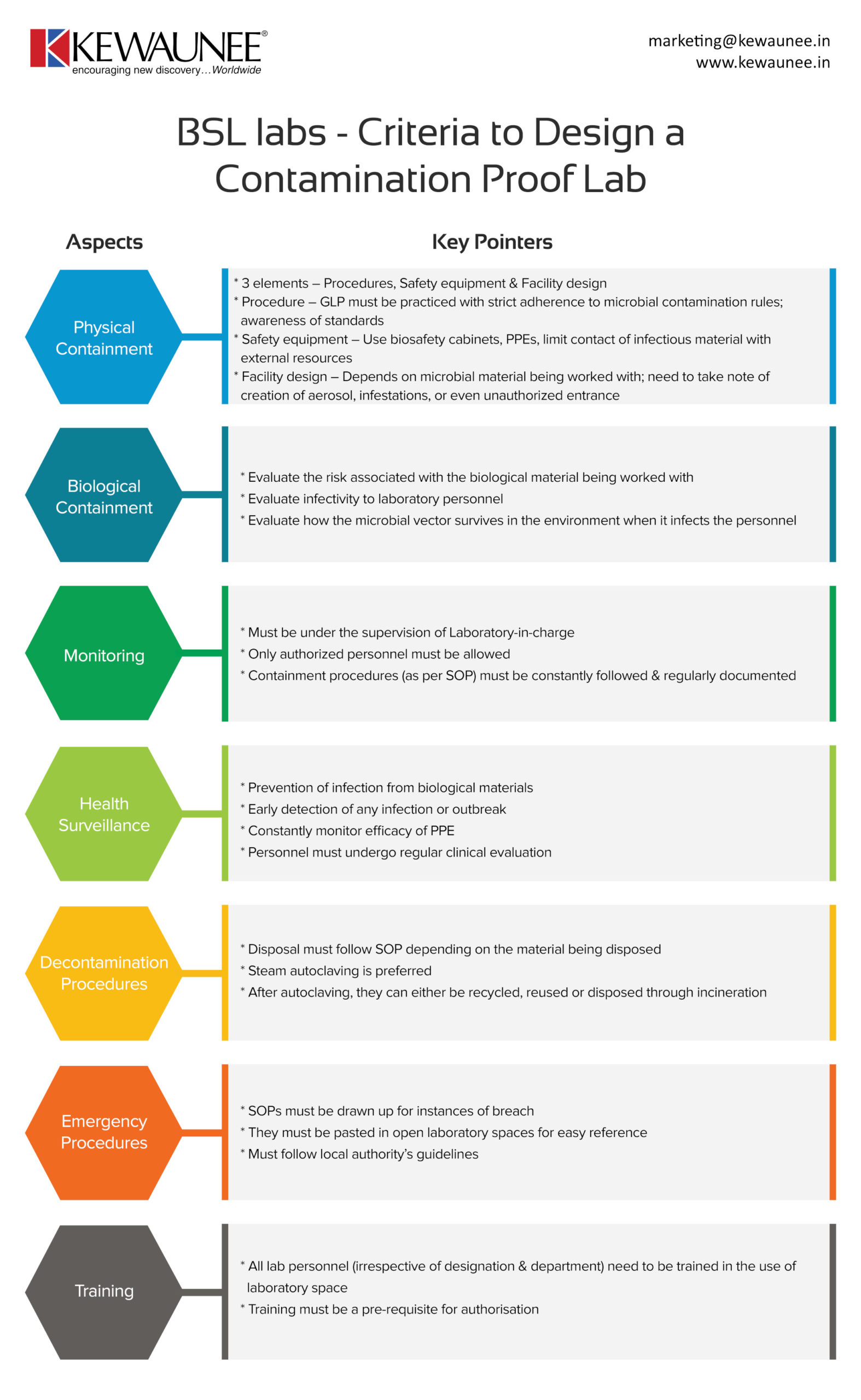
Bsl Labs Criteria To Design A Contamination Proof Lab
Biosafety laboratories are tricky to install because of the work that is being carried out. The compliance standards are much higher in this case, and there are multiple aspects to account for.
In this infographic, we’ve laid down certain things you need to account for when setting up a contamination proof BSL lab. A list of our services can be found here.
| Aspects to Consider |
Key pointers |
| Physical containment |
- 3 elements – Procedures, Safety equipment & Facility design
- Procedure – GLP must be practiced with strict adherence to microbial contamination rules; awareness of standards
- Safety equipment – Use biosafety cabinets, PPEs, limit contact of infectious material with external resources
- Facility design – Depends on microbial material being worked with; need to note creation of aerosol, infestations, or even unauthorized entrance
|
| Biological containment |
- Evaluate the risk associated with the biological material being worked with
- Evaluate infectivity to laboratory personnel
- Evaluate how the microbial vector survives in the environment when it infects the personnel
|
| Monitoring |
- Must be under the supervision of Laboratory-in-charge
- Only authorized personnel must be allowed
- Containment procedures (as per SOP) must be constantly followed & regularly documented
|
| Health Surveillance |
- Prevention of infection from biological materials
- Early detection of any infection or outbreak
- Constantly monitor efficacy of PPE
- Personnel must undergo regular clinical evaluation
|
| Decontamination procedures |
- Disposal must follow SOP depending on the material being disposed
- Steam autoclaving is preferred
- After autoclaving, they can either be recycled, reused or disposed through incineration
|
| Emergency procedures |
- SOPs must be drawn up for breach
- They must be pasted in open laboratory spaces for easy reference
- Must follow local authority guidelines
|
| Training |
- All lab personnel (irrespective of designation & department) need to be trained in using laboratory space
- Training must be a pre-requisite for authorisation
|
Kewaunee, the global leader in total laboratory solutions, empowers organisations to achieve competitive advantage through safe, efficient, and contemporary laboratories. In existence since 1906, Kewaunee powers the laboratories for over 5,000 customers in more than 100 countries.