Good Laboratory Practices For Pharmaceutical Laboratories
Steel casework is one of the most used materials in laboratory furniture. It’s preferred in most laboratory settings because of its high durability and resistance. If you’re looking to maintain a sterile environment in your laboratory, steel/ metal casework is your best option.
At Kewaunee International, we have a broad range of metal casework that meet the SEFA 8-M-2016 compliance standards and would make an excellent fit for your laboratory, no matter your needs. You can find our range of products here: https://www.kewaunee.in/metal-casework.php
| Aspects to consider |
Key Pointers |
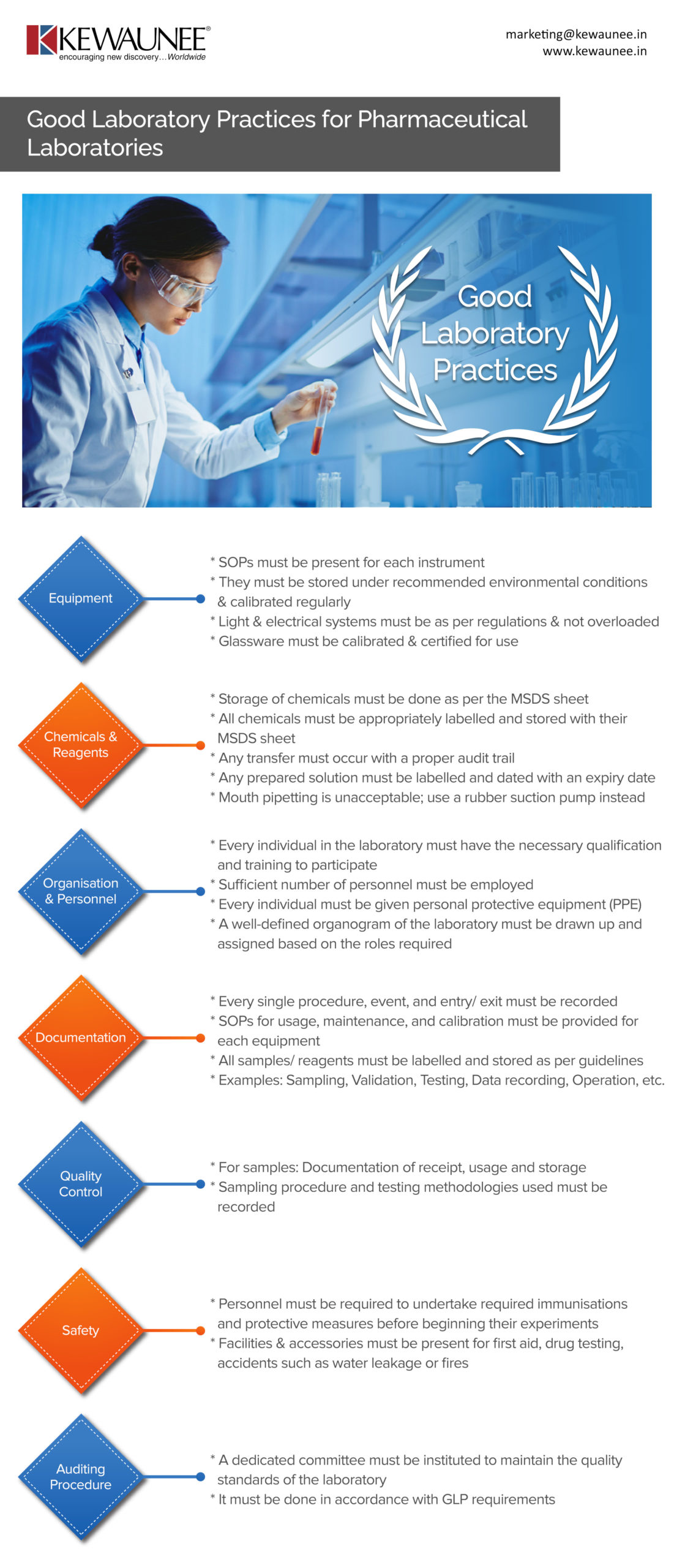
| Equipment |
- SOPs must be present for each instrument
- They must be stored under recommended environmental conditions & calibrated regularly
- Light & electrical systems must be as per regulations and not overloaded
- Glassware must be calibrated & certified for use
|
| Chemicals & Reagents |
- Storage of chemicals must be done as per the MSDS sheet
- All chemicals must be appropriately labelled and stored with their MSDS sheet
- Any transfer must occur with a proper audit trail
- Any prepared solution must be labelled and dated with an expiry date
- Mouth pipetting is unacceptable; use a rubber suction pump instead
|
| Organisation & Personnel |
- Every individual in the laboratory must have the necessary qualification and training to participate
- Sufficient number of personnel must be employed
- Every individual must be given personal protective equipment (PPE)
- A well-defined organogram of the laboratory must be drawn up and assigned based on the roles required
|
| Documentation |
- Every single procedure, event, and entry/ exit must be recorded
- SOPs for usage, maintenance, and calibration must be provided for each equipment
- All samples/ reagents must be labelled and stored as per guidelines
- Examples: Sampling, Validation, Testing, Data recording, Operation, etc.
|
| Quality Control |
- For samples: Documentation of receipt, usage, and storage
- Sampling procedure and testing methodologies used must be recorded
|
| Safety |
- Personnel must be required to undertake required immunisations and protective measures before beginning their experiments
- Facilities & accessories must be present for first aid, drug testing, accidents such as water leakage or fires
|
| Auditing Procedure |
- A dedicated committee must be instituted to maintain the quality standards of the laboratory
- It must be done in accordance with GLP requirements
|
Kewaunee, the global leader in total laboratory solutions, empowers organisations to achieve competitive advantage through safe, efficient, and contemporary laboratories. In existence since 1906, Kewaunee powers the laboratories for over 5,000 customers in more than 100 countries.